Tuesday, June 3, 2014
Sunday, June 1, 2014
Monday, February 10, 2014
Micelles Finally!
Well it has been a while since I have posted but I have been working on the problem of obtaining images of micelles using the AFM. Sprinkled in there as well were presentations and qualification exams which also took time but this received the bulk of experimental attention. I finally got somewhere after emailing Dr. S. Manne who has quite a few publications which include micelle imaging and he suggested that I was walking before I learned to crawl (paraphrasing). He suggested I try graphite first (which was the actual surface used in his paper) to get the basics down and to use contact mode to get a protocol which I could reproduce. So I acquired the materials and started learning to use contact mode, as I had not used it before. Dr. Paul Ashby gave a seminar here at the University of Oklahoma last year and gave the advice that I advance the stage and "wag" the tip to see if I had reached the surface yet. I didn't seem to get anywhere with this (I understand why now) at first but continued with this mind set in contact mode. The breakthrough came from understanding (not just using but understanding) the force curve capability of the scope. The first two figures can help explain what I mean by this. The tip is extended into the solution and if the surface is not close enough there is only interaction with the fluid surrounding the tip (air or water), which is just noise (not pictured) but when there is a surface which gives a repulsive or attractive force the curve looks something like the curve seen in the Figure 1 (air with clean silicon surface). As the tip is advanced there is an increasing repulsive force and then an attraction, or snap-to point. Then as the tip is pulled away there is a slight adhesion force which causes the force to not line up on top of the approaching line, and then there is a snap off point, the straight vertical line, as the tip suddenly leaves the surface. Now if we now add water and micelles, we have added a layer to the surface which interacts with the tip. Figure 2 shows a small blip for the approaching line, which is where the tip has broken through the micelle surface and is now at the actual surface. As the tip is pulled away there is still an adhesion force and we see a snap-off. The trick is set the imaging setpoint (the force applied to the tip which keeps its height constant) to be just before the snap-to point. Any setpoint greater than this will push right through the micelle layer and you will only see the surface, which is smooth so you will essentially see nothing (many, many, many weeks/months/years of nothing). The hardest part of most of this was translating the protocols of others using different scopes to the scope we have here at OU. Anyway, the final products are seen in Figures 3 and 4, which are deflection images (working on translating to tapping mode currently). The images show lines of micelles, which follow the grains of the surface. For CTAB on graphite these are shown to be cylindrical micelles. In order to verify this with our set-up a sharper tip will be used to check the height of the micelles. A duller tip was used because I got tired of wasting all of my good, really sharp tips on seeing nothing so I used the slightly larger tip (but not so large that I would miss the micelles if they were there). There is still a lot of work to do but now that I have finally been able to get this to work and have a protocol which I can reproduce we can move forward again instead of being stuck in once place!! Next will work on imaging micelles using tapping mode (perhaps with a tip using magnetic backing to decrease the oscillation of the water) and then start working into the PMMA trenches.
 |
| Figure 1. Force Curve in air |
 |
| Figure 2. Force curve in water with micelles on surface |
 |
| Figure 3. CTAB micelles on surface following grains (deflection) |
 |
| Figure 4. CTAB micelles on surface (deflection) |
Wednesday, October 2, 2013
A short update on cleaning and imaging procedures...
Let me be (probably not the first) one to say that if research didn't slog forward it probably wouldn't move at all. I have been working with AFM in attempts to find the right combination of AFM probe and cleaning procedure (of both the substrate and the liquid cell) which will yield micelles on the surface. I have been corresponding with contacts on campus which have experience with cleaning AFM probes (which I have mentioned before can become contaminated by the gel packing which the are stored and shipped in) but once again the difficulty of meshing schedules makes that endeavor a slow one. I am meeting with another research specialist today in hopes of looking at the set up they use and replicating their procedure and apparatus somewhere in our building so that I, and other who are trained to use the AFM, will have access to it so that cleaning of AFM probes will not be such a hassle. I was able to come up with a contamination free way of transporting probes across the university campus which will keep the probes safe, secure and clean following the cleaning, but if I am never able to get assistance in the cleaning then the container is not worth much (although I'm happy that the simple design we came up with works so well).
On another AFM front I am also spending time trying to locate the 30 nm and smaller lines which is proving to be much more difficult than the 50 nm lines I have posted about before on here. Those lines, which were much more in number creating an array that later proved to be too big for the field size and may have contributed to the lines large widths, were also slightly sloped on the side walls which caused the trench bottom to be smaller than the top (as previously discussed) and made them much easier to find. The new set of e-beam etches were not as sloped and were smaller (which is good!) are much harder to find even with smaller radii AFM tips. So far I have worked with tips down to 1 nm but from literature I have found that without cleaning of the probes the contamination of the tips might be the reason that the lines are so difficult to find (The Journal of Physical Chemistry B, 1998. 102(22): p. 4288-4294. and The Journal of Physical Chemistry C, 2008. 112(38): p. 14902-14906. and The Journal of Physical Chemistry B, 1999. 103(40): p. 8558-8567. to name a few). So once the cleaning procedure has been worked out I can tell if the lines and micelles are hard to image because of the contamination or because my procedure is still lacking. Either way I will update regarding these matters soon!
Friday, September 6, 2013
AFM is a different animal...
Because the SEM technician who runs the microscopy lab at TU has been gone I have shifted focus primarily to imaging micelles on a plain silicon surface. Although for as many papers of it I see it seems like this would be just a sit down at the AFM and get it done in a day or even a few days task, it has proven me wrong. The main issue that I have run across is that working with micelles on the AFM requires a degree of art and a degree of science. After speaking with someone this week who has worked with AFM for some time, although not on surfactants or micelles specifically, I came to realize that you really have to come to know each component that you're using personally. I have tried three tips so far that did not show promise and have moved onto a new set of three which I am hoping will at least point me in the right direction. I have also been in contact with Agilent and Nanosensors.com regarding these subjects and while they are helpful I have come to the conclusion that it will all come down to trial and error with tips and cleaning procedures. It becomes pretty frustrating when I use the AFM for several hours in a day and come away with nothing other than "this wasn't a tip that will do the job" or "that cleaning procedure left too much on the surface". However, there is always another procedure and another tip to try and with free samples from probe manufacturers I have plenty to work with. Once I find "the tip", the plan is to purchase some and coat the backside of a couple with magnetic material which could (has been shown to) increase resolution by decreasing the motion of the solvent (in our case water) by the piezo. A MAC mode nose cone will facilitate this by creating an oscillating magnetic field which will vibrate the tip alone without the piezo.
Another problem which I am dealing with is that most AFM studies done on silicon use a cleaning procedure which leaves the surface hydrophilic, usually by washing in an RCA-1 cleaning solution. For now I am just trying to achieve imaging micelles so I am following this procedure, but my work around in the end is to try a plasma cleaning step on the PMMA layered samples which should leave the exposed silicon in the trenches hydrophilic. The time of the plasma will need to be short in order to prevent damage which could alter the roughness of the exposed surfaces. This week I am trying the PPP-BSI-SPI, the PNP-TR-SPL and the SiNi AFM probes. They are all soft cantilevers (less that .1 N/m and roughly 15 kHz). The problem I have seen with these types of cantilevers so far is that the slightest environmental factor, whether it's air movement from closing the door to the room or sneezing too loudly, causes the cantilever to become erratic. I have developed a few procedural additions which help to lessen their effect and will post on the efficacy of the new tips soon.
Tuesday, July 23, 2013
False Positives
A few weeks ago I was able to travel to Tulsa to try some new E-beam lithography development procedures and the results were very promising. The new procedures, both of which used much shorter development times, were different in that one was at room temperature and the other was at -10 degrees Celsius. The results showed 20 nm trenches and even sub-20 nm trenches in some areas. At first there was some trouble viewing the samples because when they were sputtered with gold and viewed the PMMA shifted heavily and the movement could be seen to happen in real time under the SEM. This lead to the theory that if the V shape, which I have discussed in previous posts, was occurring before the sputter coating then perhaps vertical walled trenches were not having the side walls coated effectively. The samples were brought back to OU and placed in a rotating thermal evaporator and then viewed. The PMMA was motionless, otherwise moved very slowly, and the trenches were small in width as can be seen in Figures 1, 2 and 3 . One side of the wheels were larger than the other which lead me to believe that there may have been a stigmation issue.
However, when trying to replicate the results just recently I saw the larger line widths between 30 and 50 nm that I have gotten in the past, seen in Figure 4.
When discussing with Mr. Nabity he thinks that the wedges of the wheels may have shifted causing one side to be smaller in width than the other and not just a stigmation as I originally thought which is supported by the left side of the wheel being wider than the right, shown in Figure 2. However, because all wheels in the exposure are shifted is seems that it is more than just shifting as I thought if shifting had occurred it would have only been one or two wheels but not all of them. Attempts will continue to improve the resolution and straight wall profile of the trenches but much more focus will be put on imaging micelles using AFM, which is still moving along slowly.
I received some free samples from an AFM probe company and have been trying to coax high resolution images out of them but have been having some difficulty. Part of the problem, I believe, is that all of the literature I have read states that they used cantilevers with very low resonant frequencies and force constants, which makes sense because I am trying to see "soft" things (micelles). However, some of the probes are so flexible is seems that even after centering the laser off of the cantilever onto the photodetector they are prone to drift heavily. Also, the autotune function of the AFM has trouble picking up the low resonant frequencies so manual tuning procedures are going to be done in order to work around this.
Another issue that I have found is that apparently the gel packs in which the AFM tips are stored and shipped cause contamination. I am trying to arrange a way of cleaning the tips in an ozone chamber on campus here at OU and depending on scheduling I am hopeful that I can get this done sometime in the next week!
All in all, still working on the trenches but I believe that they are at a good place where I can start using them for initial results once I can image micelles on the silica surface.
 |
| Figure 1. Trenches developed June 27th using Cold development with shortened time |
 |
| Figure 2. Trenches developed June 27th using Cold development with shortened time |
 |
| Figure 3. Trenches developed June 27th using Cold development with shortened time |
 |
| Figure 4. Trenches developed July 18th using Cold development with shortened time |
Monday, July 1, 2013
Minor Successes Can Lead to Great Victories...(Metal Lift-Off and Hi-Res AFM Probes)
In the world of science I have come to learn that with every small step forward you gain a tool or skill that will make research easier and more efficient in the future. At times I feel like I'm not really moving forward, but then I remember that a marathon is not completed in a single event but many steps. Lately I have had two small victories in the areas of E-beam lithography and AFM. The first is that upon completion of a metal life-off procedure the lines created in the PMMA are actually 20 nm or less! Images of these lift-offs can be seen in Figures 1 and 3. The problem that we continue to battle is that the side walls are not straight up and down vertical but at a slant. At TU last week I used a much shorter development time which lead to the very small metal lift-off lines. When the PMMA was viewed in the SEM, however, it was seen to be continuously pulling away (trenches were widening) due to beam damage.
This raises the question of whether or not we have actually been reaching the small line widths before and the SEM viewing was distorting the PMMA or if they were wide and then viewed. I believe the latter to be true because this was the first instance where the PMMA was seen to be actively moving under the electron beam, whereas before it had already moved and was then viewed. I think that the longer development times overdeveloped the walls, causing the slant, which were then viewed under SEM and did not move because they had already reached their "equilibrium" position. So when the development time was shortened only the exposed area was developed and upon viewing the PMMA began shifting to its equilibrium position and we were able to see it.
Another question that arises is how it the beam damage occurring if we are sputtering with gold? Two possibilities is that the gold layer is not thick enough (roughly 2.5 nm on the surface may not be enough to negate the effects of the beam) or perhaps the side walls of the trench were not coated because they were straight up and down. If this were the case if could also explain why the overdeveloped slanted walls did not move when viewed. Because they were already slanted the gold formed a layer there which could have helped protect against beam damage. The straight vertical walls may not be coated as well and therefore when viewed under SEM they are not protected and experience the beam damage. This theory will be checked using a rotating thermal evaporator in an attempt to coat the side walls of an un-viewed sample, and it will then be viewed to see if the same damaged and extreme movement occurs again.
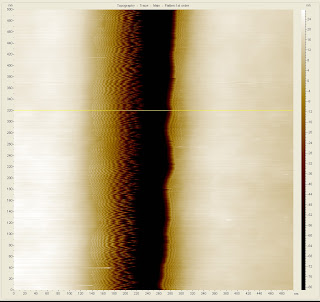
The second small victory is the use of high-resolution AFM probes in order to see the bottom of one of our trenches and make a more accurate measurement. The cross sectional view of such a trench can be seen in Figure 4. As compared with the last post in which the trench was roughly measured as 300 nm across and "V" shaped, this new measurement shows a trench with a square bottom. The slanted walls are still present, and show some tip-sample interaction on the left side of the cross section which is still being investigated, but the sample bottom appears to be roughly 58 nm across. The sample used was an older one with many doses being tested and it is difficult to know which dosage this was but due to the recent success with the metal lift-off procedure a usable dosage is much more easily identified.
The next goal is to obtain micelle images on a silicon surface using high-resolution AFM probes, along with the work being done to check how vertical the walls of the trenches are and perhaps obtain an image of them using SEM if possible.
This raises the question of whether or not we have actually been reaching the small line widths before and the SEM viewing was distorting the PMMA or if they were wide and then viewed. I believe the latter to be true because this was the first instance where the PMMA was seen to be actively moving under the electron beam, whereas before it had already moved and was then viewed. I think that the longer development times overdeveloped the walls, causing the slant, which were then viewed under SEM and did not move because they had already reached their "equilibrium" position. So when the development time was shortened only the exposed area was developed and upon viewing the PMMA began shifting to its equilibrium position and we were able to see it.
Another question that arises is how it the beam damage occurring if we are sputtering with gold? Two possibilities is that the gold layer is not thick enough (roughly 2.5 nm on the surface may not be enough to negate the effects of the beam) or perhaps the side walls of the trench were not coated because they were straight up and down. If this were the case if could also explain why the overdeveloped slanted walls did not move when viewed. Because they were already slanted the gold formed a layer there which could have helped protect against beam damage. The straight vertical walls may not be coated as well and therefore when viewed under SEM they are not protected and experience the beam damage. This theory will be checked using a rotating thermal evaporator in an attempt to coat the side walls of an un-viewed sample, and it will then be viewed to see if the same damaged and extreme movement occurs again.
The second small victory is the use of high-resolution AFM probes in order to see the bottom of one of our trenches and make a more accurate measurement. The cross sectional view of such a trench can be seen in Figure 4. As compared with the last post in which the trench was roughly measured as 300 nm across and "V" shaped, this new measurement shows a trench with a square bottom. The slanted walls are still present, and show some tip-sample interaction on the left side of the cross section which is still being investigated, but the sample bottom appears to be roughly 58 nm across. The sample used was an older one with many doses being tested and it is difficult to know which dosage this was but due to the recent success with the metal lift-off procedure a usable dosage is much more easily identified.
The next goal is to obtain micelle images on a silicon surface using high-resolution AFM probes, along with the work being done to check how vertical the walls of the trenches are and perhaps obtain an image of them using SEM if possible.
 |
| Figure 1. Metal Lift-Off using Gold with a PMMA mask. Line widths are sub 20 nm |
 |
| Figure 2. Metal Lift-off Using Gold with PMMA Mask showing Trench Widths Sub 20 nm |
 |
| Figure 3. AFM Scan of PMMA trench made by E-Beam Exposure |
 |
| Figure 4. Cross Section of PMMA Trench Obtained using High-Resolution AFM Probes |
Subscribe to:
Posts (Atom)